
The question-and-answer on valence and valence electrons is fully discussed below. Question Answer on valency and valence electrons For this, carbon always forms covalent bonds by mutually sharing its own 4 electrons. Because in this case, you have to give a lot of energy. Because it never becomes a c -4or c +4ion by accepting or rejecting the 4 electrons in the last cell in order to gain its stability. In order for this element to be stable, the electrons in its last cell have to be mutually shared with other elements.Īn example is a carbon. There are some elements that can neither gain nor lose electrons in their last cell. Many of you may be confused as to where the word mutual share in definition came from. Thus, the valency of carbon is four.Īfter each element gains stability, its electronic configuration will be like the nearest Nobel gas.

What is Valancy of CarbonĪccording to the octet theory, in order for each element to reach a stable state, the number of electrons it leaves, or gain or mutual shares in order to fill its octet, we will call that element the valence. So, for this, you need to understand the difference between valency and valence electrons. So look at this illustration of the electric configuration of carbon.Īlso, many of you are confused between the valency of carbon and valence electron. The first cell has two electrons and the last cell has four electrons. If you look at the electronic configuration of carbon, you can see that there are two cells present in carbon.
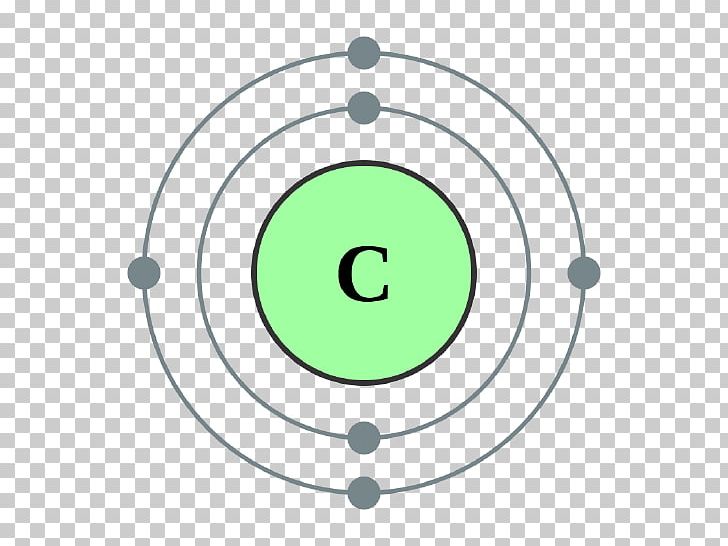
That is, there are 4 electrons present in the last cell of carbon. The above two questions mean the same thing, so the carbon has four valence electrons.


 0 kommentar(er)
0 kommentar(er)
